View the original news release.
Plants secrete organic compounds such as amino acids or enzymes known as root exudates, which are key in recruiting and selecting relevant beneficial microbes to colonize in the rhizosphere. Studying these microbial-plant interactions is critical to better understand the relationship between plant growth and the role soils play in keeping carbon from the atmosphere.
A recent study led by EESA scientists published in ISME Communications investigated microbial colonization along different parts of the roots of young Brachypodium plants–a plant type used as a model for grasses that produce biomass, biofuel, and feed. The study was part of the m-CAFEs Science Focus Area project and improves what is known about key factors involved in microbial colonization in the rhizosphere.
The team also included scientists from Berkeley Lab’s Biosciences Area, Trent Northen and Pete Andeer, and UC Berkeley researchers Spencer Diamond and Jill Banfield, as well as former EESA Postdocs Shwetha Acharya, Mon Oo Yee, Nameera Baig and Omolara Aladesanmi
“Our understanding of the soils and soil microbes surrounding the bottom half of a plant is limited since it’s invisible belowground,” said Staff Scientist Romy Chakraborty, who led the research effort. “Our findings help to put the pieces together of how the rhizosphere functions and interacts with the microbes that surround it–even in very young 2-week-old plants.”
The m-CAFEs researchers studied microbial communities at different zones of the roots in three different types of growth containers: conventional pots, tubes, and standardized fabricated ecosystems (EcoFABs), designed to mimic conditions of a natural environment. The goal was to characterize microbial communities on the tips of the roots, the soil at the base of the roots and regular bulk soil that was not in the rhizosphere environment. The researchers incorporated different growth container types to see if the different shapes or sizes influenced finer scale plant-microbe interactions in smaller, younger plants often used for research.
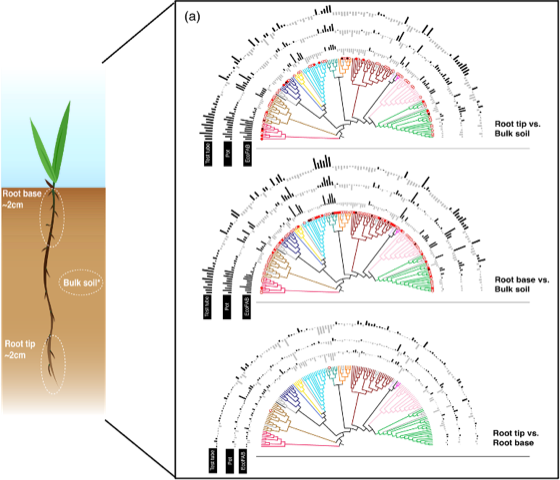
The team found that the microbes found in the bulk soil compared to the microbes in the rhizosphere were distinct from each other, indicating that microbes are recruited from bulk soil outside of the rhizosphere onto the root surface–as early as 14 days into plant growth.This is possibly due to the effect of root exudation, in which roots release substances such as amino acids and enzymes that attract more microbial communities than environments outside of the rhizosphere.
Another key finding was that genes associated with different metabolic pathways and root colonization were more abundant in root tips than those associated with nutrient-limitations and environmental stress, implying the absence of easily available and stable carbon and nutrients in bulk soil relative to roots. The study also shows that using different growth containers with different shapes, volumes and sizes had no observed effect on the microbial composition throughout the roots.
“The root exudates, the substances released by roots, along different parts of roots is considered an important parameter in rhizosphere dynamics, systematic and standardized studies probing this deeper are lacking especially in the early critical stages of plant growth,” said Chakraborty.
This study reveals key information on rhizosphere microbiology, which is a hotbed of carbon and nutrient transformations in all ecosystems. Closing the knowledge gap related to this environment and the microbes associated with it is essential to building our understanding of biogeochemical and carbon cycling.
